What do we do?
Discover and engineer enzymes for biotechnological applications
Nature’s vast reservoir of microorganisms constitutes an unfathomable wealth of enzymes, which can perform all kinds of reactions useful for the industry. However, screening through the natural or man-made genetic diversity to find these proteins is an overwhelming task.
In the HT Discovery Lab, we develop methods to find and engineer industrially relevant enzymes in a large metagenomic or randomized libraries as fast and efficiently as possible.

Why do we do it?
To make industrial reactions more sustainable
The use of enzymes to catalyze industrial reactions, known as “biocatalysis”, has the potential to make chemical processes more sustainable and compliant with the principles of Green Chemistry. Their application in industrial processes and everyday consumer products will help us achieve the EU goals towards sustainability and the developing bioeconomy, moving away from oil-based chemistry.
How do we do it?
Using (ultra)high-throughput screening methods
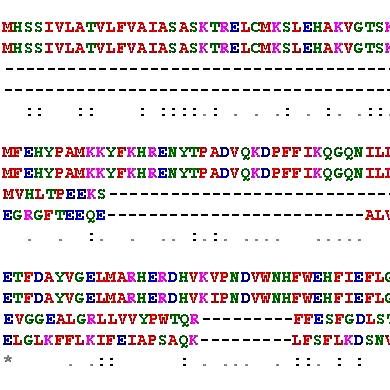
Biological selections are inexpensive methods to find enzymes that couple the improved fitness of a protein to the survival of a biological host under selective pressure. In the HT Discovery Lab, we investigate the accuracy of biological selections to enhance the stability of enzymes to withstand harsh conditions of industrial processes, such as the presence of organic cosolvents or high temperatures. However, the complexity of cellular metabolism limits the properties that can be selected.
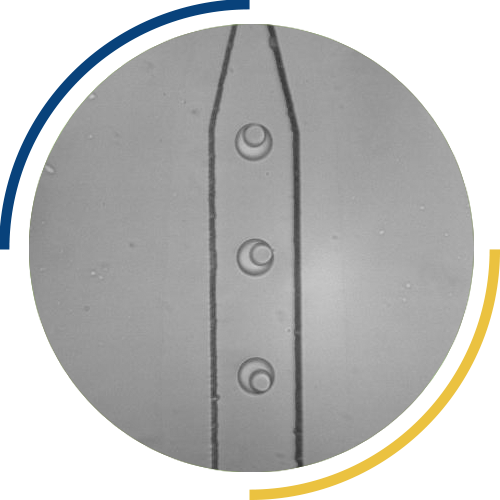
For this reason, we also develop screening methods, which involve individual enzymatic assays in vitro of each individual enzyme. To shorten this long, tedious and expensive process, droplet microfluidics enables the miniaturization of assays with throughput of kHz rates as well as a 1000x reduction of volume and assay costs.
Finally, once suitable enzymes have been found, we study what has made them better and more robust, thus uncovering how and why enzymes function and ultimately, learning the language of proteins.

What we do? Discover and engineer suitable enzymes
Nature’s vast reservoir of microorganisms constitutes an unfathomable wealth of enzymes, which can perform all kinds of reactions useful for the industry. However, sifting through the natural or man-made genetic diversity to find and harvest these molecules is an overwhelming task. Existing robotic screening tools are relatively slow, extremely expensive and largely inaccessible to most researchers.
In the HT discovery lab, we develop methods to find and engineer industrially relevant enzymes in metagenomes or randomized libraries as fast and efficiently as possible.

Why we do it? to make industrial reactions more sustainable.
The use of enzymes to catalyse industrial reactions, known as “biocatalysis”, has the potential to make chemical processes more sustainable and compliant with the principles of Green Chemistry. Their application for everyday consumer products will help us achieve the EU goals towards sustainability and the developing bioeconomy, moving away from oil-based chemistry. This highlights the importance of fostering the way from the lab to the industrial application.

How do we do it? using (ultra)high-throughput screening methods.
Biological selections are inexpensive methods to find enzymes that couple the improved fitness of a protein to the survival of a biological host under selective pressure. In the HT Discovery Lab, we investigate the accuracy of selections to enhance the stability of enzymes to withstand harsh conditions of industrial processes, such as the presence of organic cosolvents or high temperatures. However, the complexity of cellular metabolism limits the properties that can be selected.
For this reason, we also work on screening methods, which involve individual enzymatic assays in vitro of each enzyme variant generated. To shorten this long, tedious and expensive process, droplet microfluidics provides the required miniaturization of assays with throughput of kHz rates as well as a 1000x reduction of volume and assay costs. Moreover, microfluidics enables the conversion of general lab operations (additions, aliquoting, detection of a given property) into a particular chip design.
Finally, after suitable enzymes are found, we study what makes them better and more robust, thus uncovering how and why enzymes do what they do, that is, we learn the language of proteins.
Research lines
In the lab, we carry out research in 3 different fronts, all with the aim to discover and engineer enzymes to support the circular bioeconomy. These 3 research lines can intersect one another, which makes our work even richer.
Funding
Ongoing projects

BLUETOOLS
Innovative tools for sustainable exploration of marine microbiomes: Towards a circular blue economy and healthier marine environments
Website
Duration: 01/12/2022 – 30/1/2026
Budget: 7,623,032.50€
Funding Programme: HORIZON EUROPE
Participation: Coordinator

RadicalZ
Rapid discovery and development of enzymes for novel and greener consumer products
Website
Duration: 01/06/2021 – 31/05/2025
Budget: 6,004,308.75 €
Funding Programme: HORIZON 2020
Participation: Coordinator

CC-TOP
C-C Bond Formation Using Top Performing Enzymes
Website
Duration: 01/03/2021 – 28/02/2025
Budget: 3,971,466.72€
Funding Programme: HORIZON 2020
Participation: Beneficiary
Previous projects

MetaFluidics
Advanced toolbox for rapid and cost-effective functional metagenomic screening – microbiology meets microfluidics
Website
Duration: 01/06/2016 – 31/07/2020
Funding: 8.808.376,75 €
Funding Programme: HORIZON 2020
Participation: Coordinator

CarbaZymes
Sustainable industrial processes based on a C-C bond-forming enzyme platform
Website
Duration: 01/04/2014 – 31/03/2018
Funding: 8.213.921 €
Funding Programme: HORIZON 2020
Participation: IP

HotDrops
Ultra High-throughput platform for the selection of thermostable proteins by thermophilic in vitro transcription-translation and microfluidics”. Marie Curie Industrial and academic partnerships and pathways
Website
Duration: 01/06/2013 – 31/05/2017
Funding: 1.973.797 €
Funding Programme: SEVENTH FRAMEWORK PROGRAMME
Participation: Team member
Publications
From accurate genome sequence to biotechnological application: The thermophile Mycolicibacterium hassiacum as experimental model. , , , , & Microbial Biotechnology, 17, e14290. (2024)
Ultrahigh-Throughput Screening of Metagenomic Libraries Using Droplet Microfluidics. Cecchini, D.A., Sánchez-Costa, M., Orrego, A.H., Fernández-Lucas, J., Hidalgo, A. In: Magnani, F., Marabelli, C., Paradisi, F. (eds) Enzyme Engineering. Methods in Molecular Biology, vol 2397. Humana, New York, NY, 2022.
https://doi.org/10.1007/978-1-0716-1826-4_2
Ruminal microbiota as a source of industrial enzymes. Hervás, G., Toral, P. G., Blas, L., Hidalgo, A., Frutos, P., Albéitar, 254: 12-16, 2022
https://digital.csic.es/handle/10261/269441
A Coupled Ketoreductase-Diaphorase Assay for the Detection of Polyethylene Terephthalate-Hydrolyzing Activity. M. Gimeno-Pérez, J. D. Finnigan, C. Echeverria, S. J. Charnock, A. Hidalgo, D. M. Mate, ChemSusChem 2022, 15, e202102750.
https://doi.org/10.1002/cssc.202102750
Flow-based method for biofilm microbiota enrichment and exploration of metagenomes. Hageskal, G., Heggeset, T.M.B., Nguyen, GS. et al. AMB Expr 12, 36 (2022).
Thermostability Engineering of a Class II Pyruvate Aldolase from Escherichia coli by in Vivo Folding Interference. Sandra Bosch, Esther Sanchez-Freire, María Luisa del Pozo, Morana C̆esnik, Jaime Quesada, Diana M. Mate, Karel Hernández, Yuyin Qi, Pere Clapés, Đurđa Vasić-Rački, Zvjezdana Findrik Blažević, José Berenguer, and Aurelio Hidalgo; ACS Sustainable Chemistry & Engineering 2021 9 (15), 5430-5436 DOI:10.1021/acssuschemeng.1c00699
https://pubs.acs.org/doi/full/10.1021/acssuschemeng.1c00699
Insights into the molecular determinants of thermal stability in halohydrin dehalogenase HheD2. Wessel, J., Petrillo, G., Estevez-Gay, M., Bosch, S., Seeger, M., Dijkman, W.P., Iglesias-Fernández, J., Hidalgo, A., Uson, I., Osuna, S. and Schallmey, A. (2021), FEBS J, 288: 4683-4701. DOI:10.1111/febs.15777
https://febs.onlinelibrary.wiley.com/doi/full/10.1111/febs.15777
Biochemical and Structural Characterization of a novel thermophilic esterase EstD11 provide catalytic insights for the HSL family; Vega Miguel-Ruano, Ivanna Rivera, Jelena Rajkovic, Kamila Knapik, Ana Torrado, José Manuel Otero, Elisa Beneventi, Manuel Becerra, Mercedes Sánchez-Costa, Aurelio Hidalgo, José Berenguer, María-Isabel González-Siso, Jacobo Cruces, María L. Rúa, Juan A. Hermoso, Computational and Structural Biotechnology Journal, Volume 19, 2021, Pages 1214-1232,
Thermostability enhancement of the Pseudomonas fluorescens esterase I by in vivo folding selection in Thermus thermophilus.Mate DM, Rivera NR, Sanchez-Freire E, Ayala JA, Berenguer J and Hidalgo A. Biotechnology and Bioengineering. 2020; 117(1), 30-38.
https://onlinelibrary.wiley.com/doi/full/10.1002/bit.27170
Hypoxanthine-Guanine Phosphoribosyltransferase/adenylate Kinase From Zobellia galactanivorans: A Bifunctional Catalyst for the Synthesis of Nucleoside-5′-Mono-, Di-and Triphosphates. Acosta, J., Del Arco, J., Del Pozo, M.L., Herrera-Tapias, B., Clemente-Suárez, V.J., Berenguer, J., Hidalgo, A., Fernández-Lucas, J. Front.Bioeng. Biotechnol. 2020; 8, 677, DOI: 10.3389/fbioe.2020.00677
Intraparticle pH Sensing Within Immobilized Enzymes: Immobilized Yellow Fluorescent Protein as Optical Sensor for Spatiotemporal Mapping of pH Inside Porous Particles. In: Immobilization of Enzymes and Cells. Consolati, T., Bolivar, J. M., Petrasek, Z., Berenguer, J., Hidalgo, A., Guisan, J. M., Nidetzky, B. Springer. 2020; pp 319-333.
Stabilization of Multimeric Enzymes via Immobilization and Further Cross-Linking with Aldehyde-Dextran. Mateo, C.; Pessela, B.C.C.; Fuentes, M.; Torres, R.; Betancor, L.; Hidalgo, A.; Fernandez-Lorente, G.; Fernandez-Lafuente, R.; Guisan, J.M., Methods in Molecular Biology; 2020 | Book chapter
A modular vector toolkit with a tailored set of thermosensors to regulate gene expression in Thermus thermophilus. Verdú C, Sanchez-Freire E, Ortega C, Hidalgo A, Berenguer J* and Mencía M*. ACS Omega. 2019 Aug 27;4(11):14626-14632.
doi: 10.1021/acsomega.9b02107
https://pubmed.ncbi.nlm.nih.gov/31528818/
A modular vector toolkit with a tailored set of thermosensors to regulate gene expression in Thermus thermophilus. Verdú, C., Sanchez, E.; Ortega, C.; Hidalgo, A.; Berenguer, J.; Mencía, M. ACS Omega. 2019; 4, 14626-14632. doi: 10.1021/acsomega.9b02107
Functional characterization and structural analysis of NADH oxidase mutants from Thermus thermophilus HB27. Role of residues 166, 174, and 194 in the catalytic properties and thermostability. Rocha-Martin, J., Sánchez-Murcia, P.A., López-Gallego, F., Hidalgo, A., Berenguer, J., Guisan, J. M. Microorganisms. 2019; 7, 515. doi: 10.3390/microorganisms7110515
A single mutation in cyclodextrin glycosyltransferase from Paenibacillus barengoltziichanges cyclodextrin and maltooligosaccharides production. Castillo J, Caminata Landriel S, Sánchez Costa M, Taboga OA, Berenguer J, Hidalgo A, Ferrarotti SA, Costa H. Design and Selection, 2018; 31(10), 399-407. doi: 10.1093/protein/gzy034
https://pubmed.ncbi.nlm.nih.gov/30690526/
Bio-based pH internally sensitive materials: immobilized yellow fluorescent protein as optical sensor for spatiotemporal mapping of pH inside porous matrices.Consolati T, Bolívar JM, Petrasek Z, Berenguer J, Hidalgo A, Guisán JM, and Nidetzky B.
ACS Appl. Mater. Inter. 2018; 10: 6858-6868. doi: 10.1021/acsami.7b16639
https://pubs.acs.org/doi/abs/10.1021/acsami.7b16639
A brief guide to the high-throughput expression of directed evolution libraries. Ribeiro AL, Mencía M and Hidalgo A.
Methods M Biol. 2018; 1685: 131-143. doi: 10.1007/978-1-4939-7366-8_7
https://link.springer.com/protocol/10.1007/978-1-4939-7366-8_7
Stabilization of enzymes by using thermophiles. Ribeiro AL, Sánchez M, Hidalgo A, and Berenguer J
Methods Mol. Biol. 2017; 1465: 297-312. doi: 10.1007/978-1-4939-7183-1_21
https://pubmed.ncbi.nlm.nih.gov/28710637/
Are in vivoselections on the path to extinction? Berenguer J, Mencía M, and Hidalgo A
Biotechnol. 2017; 10: 46-49. doi: 10.1111/1751-7915.12490
https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1111/1751-7915.12490
Stabilization of Enzymes by Using Thermophiles MICROBIAL STEROIDS: METHODS AND PROTOCOLS. Ana Luisa Ribeiro, Mercedes Sánchez, Aurelio Hidalgo, José Berenguer
ISSN 1064-3745, ISBN 978-1-4939-7182-4. doi:10.1007/978-1-4939-7183-1_21
https://link.springer.com/protocol/10.1007/978-1-4939-7183-1_21
Erratum to: A novel thermostable protein-tag: optimization of the Sulfolobus solfataricus DNA-alkyl-transferase by protein engineering. Vettone A, Serpe M, Hidalgo A, Berenguer J, Del Monaco G, Valenti A, Rossi M, Ciaramella M and Perugino G. Extremophiles, 2016; 20(1), 15-17.
Extremophiles 2016, 20: 1-13.
One-pot Simple methodology for CAssette Randomization and Recombination for focused directed evolution (OSCARR).Hidalgo A, Schließmann A and Bornscheuer UT
Mol. Biol. 1179: 207-212. doi: 10.1007/978-1-4939-1053-3_14
https://link.springer.com/protocol/10.1007/978-1-4939-1053-3_14
Parallel pathways for nitrite reduction during anaerobic growth in Thermus thermophilus. Laura Alvarez, Carlos Bricio, Aurelio Hidalgo, José Berenguer
Journal of Bacteriology. 2014; 196, pp.1350-1358. DOI:1128/JB.01042-13
https://jb.asm.org/content/196/7/1350.short
Transferable denitrification capability of Thermus thermophilus. Laura Alvarez, Carlos Bricio, Alba Blesa, Aurelio Hidalgo, José Berenguer
Applied Environ. 2014; Microbiol.80, pp.19-28. DOI: 10.1128/AEM.02594-13
https://aem.asm.org/content/80/1/19.short
One-pot, Simple Methodology for Cassette Randomization and Recombination for Focused Directed Evolution (OSCARR) Directed Evolution Library Creation: Methods and protocols. Aurelio Hidalgo, Anna Schließmann, Uwe T Bornscheuer
Methods in Molecular Biology. EMJ Gillian, JN Copp y DF Ackerley (Eds). pp.207-212. DOI: 10.1007/978-1-4939-1053-3_14
Biotechnological applications of Thermus thermophilusas host. Hidalgo A and Berenguer J
Biotechnol. 2013; 2: 304-312. doi: 10.2174/18722083113076660030
https://www.ingentaconnect.com/content/ben/cbiot/2013/00000002/00000004/art00005
Engineering the substrate specificity of a thermophilic penicillin acylase from Thermus thermophilus.Torres LL, Cantero A, Del Valle M, Marina A, Lopez-Gallego F, Guisan JM, Berenguer J and Hidalgo A
Environ. Microbiol. 2013; 79: 1555-1562. doi:10.1128/AEM.03215-12
Functional expression of a penicillin acylase from the extreme thermophile Thermus thermophilusHB27 in Escherichia coli. Torres LL, Ferreras ER, Cantero A, Hidalgo A and Berenguer J
Cell Fact. 2012; 11: 105. doi: 10.1186/1475-2859-11-105.
https://link.springer.com/article/10.1186/1475-2859-11-105
Promiscuous enantioselective (-)-γ-lactamase activity in the Pseudomonas fluorescensesterase I. Torres LL, Schließmann A, Schmidt M, Silva-Martin N, Hermoso JA, Berenguer J, Bornscheuer UT and Hidalgo A
Biomol. Chem. 2012; 10: 3388-3392. doi:10.1039/C2OB06887G.
https://pubs.rsc.org/en/content/articlelanding/2012/ob/c2ob06887g/unauth#!divAbstract
Characterization and further stabilization of a new anti-Prelog specific alcohol dehydrogenase from Thermus thermophilus HB27 for asymmetric reduction of carbonyl compounds. Javier Rocha-Martín, Daniel Vega, Juan M Bolivar, Aurelio Hidalgo, José Berenguer, José M Guisán, Fernando López-Gallego
Bioresource Technol. 2012; 103, pp.343-350. doi:1016/j.biortech.2011.10.018
https://www.sciencedirect.com/science/article/abs/pii/S0960852411014647
The residue 179 is involved in product specificity of the Bacillus circulans DF9R cyclodextrin glycosyltransferase. Hernán Costa, Ana Julia Distéfano, Cristina Marino-Busjle, Aurelio Hidalgo, José Berenguer, Mirtha Biscoglio de Jiménez Bonino, Susana Alicia Ferrarotti
Applied Microbiology and Biotechnology. 2012; 94, pp.123-130. doi: 10.1007/s00253-011-3623-6
Cloning, functional expression, biochemical characterization and structural analysis of a haloalkane dehalogenase from Plesiocystis pacifica SIR-1. Hesseler, Martin; Bogdaovic, Xenia; Hidalgo, Aurelio, Palm, Gottfried J.; Hinrichs, Winfried; Bornscheuer, Uwe T.; Berenguer, José
Applied Microbiology and Biotechnology. 2011; 91, pp.1049-1060. DOI: 1007/s00253-011-3328-x
https://link.springer.com/article/10.1007/s00253-011-3328-x
Modulation of the distribution of small proteins within porous matrixes by smart-control of the immobilization rate. Juan M Bolivar, Aurelio Hidalgo, Lucia Sánchez-Ruiloba, José Berenguer, José M Guisán, Fernando López-Gallego
Journal of Biotechnology. 155, pp.412-420. DOI: 10.1016/j.jbiotec.2011.07.039
https://www.sciencedirect.com/science/article/pii/S0168165611004524
New biotechnological perspectives of a NADH oxidase variant from Thermus thermophilus HB27 as NAD+-recycling enzyme BMC. Javier Rocha-Martín, Daniel Vega, Juan M Bolivar, cesar A Godoy, Aurelio Hidalgo, José Berenguer, José M Guisán, Fernando López-Gallego
BMC Biotechnology. 2011; 11, pp.101. doi: 10.1186/1472-6750-11-101
Suppression of water as a nucleophile in Candida antarctica lipase B catalysis. Larsen, Marianne Wittrup; Zielinska, Dorota F.; Martinelle, Mats; Hidalgo, Aurelio; Jensen, Lars Juhl; Bornscheuer, Uwe T.; Hult, Karl
2010; 11, pp.796-801 DOI: 10.1002/cbic.200900743
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/cbic.200900743
Increased Enantioselectivity by Engineering Bottleneck Mutants in an Esterase from Pseudomonas fluorescens. Anna Schliessmann, Aurelio Hidalgo, José Berenguer, Uwe T Bornscheuer
(Cover) ChemBioChem. 10, pp.2920-2923. DOI: 10.1002/cbic.200900563
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/cbic.200900563
Thermus thermophilus as a biological model. Felipe Cava, Aurelio Hidalgo, José Berenguer
13, pp.213-231 doi: 10.1007/s00792-009-0226-6